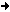The "eutectic point" is that particular mixture of two or more
substances that has the lowest melting point. Tin melts at 232 C,
lead at 327 C. For tin-lead solder it's 63%-37%, melting at around
183 C. A chart of the melting points of tin-lead alloys looks
something like this:
deg. C
327 +
300 +
275 +
250 +
225 + +
200 + +
175 +
% Sn 0 10 20 30 40 50 60 70 80 90 100
% Pb 100 90 80 70 60 50 40 30 20 10 0
Sn is tin; Pb is lead.
Because tin is generally far more expensive than lead, unscrupulous
manufacturers have been known to sell 37% Sn / 63% Pb solder to those
looking for the opposite mixture.
My source for the melting points quoted above is the 22nd edition
(1937) of the Handbook of Chemistry and Physics. I've had to do some
guessing, though, because the book contains conflicting data in various
tables, and does not give the melting point for the eutectic alloy,
which I have supplied from memory.
Peter Neilson
[ Good memory ! See http://www-053.connix.com/amt/alloy.html
[ which lists eutectic temperatures for popular solder alloys.
[ The conflicting data probably results from different definitions
[ of "melted". -- Robbie
|